Recent Publications





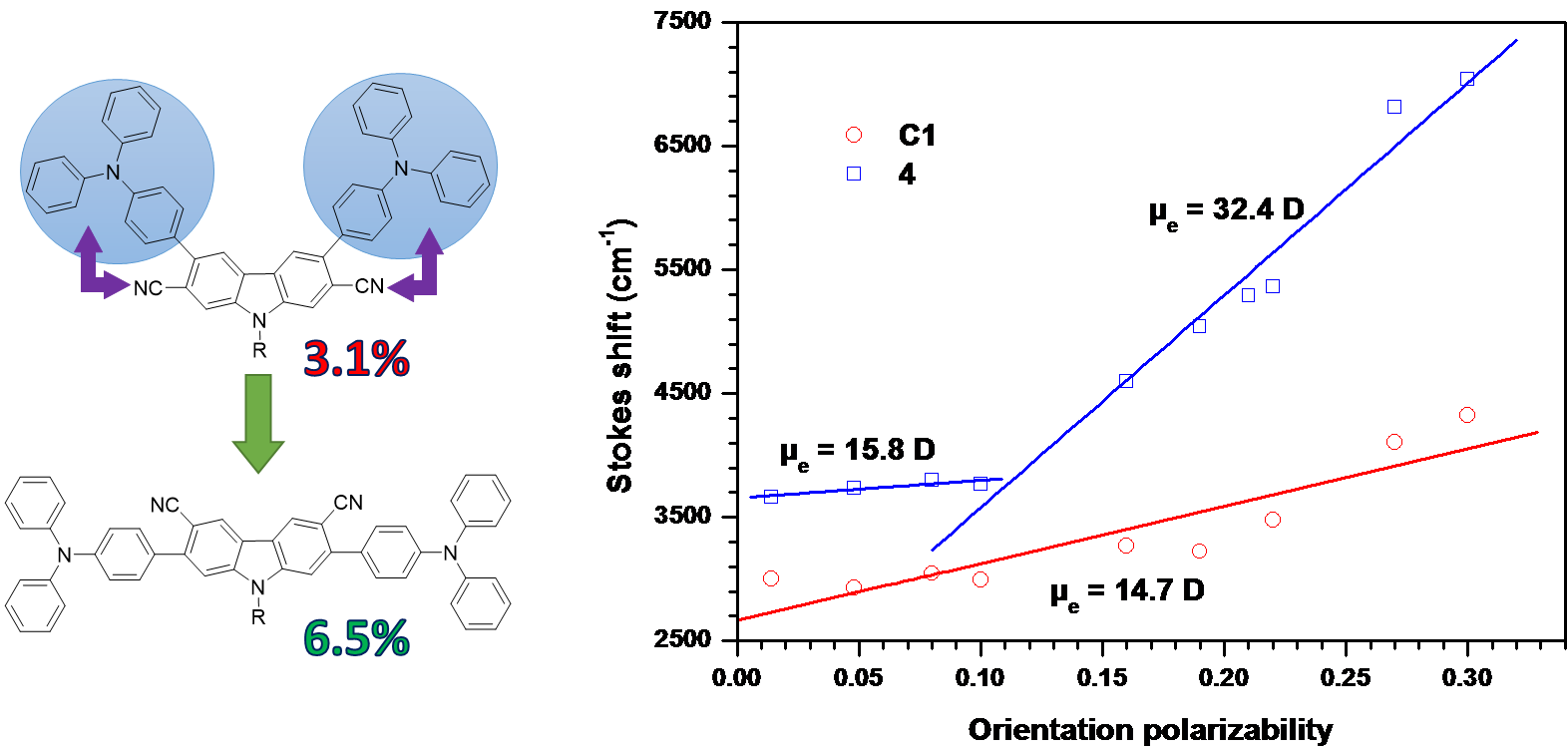
Polarity tuning of fluorene derivatives by chromophores to achieve efficient blue electroluminescent materials, K. R. J. Thomas, A. Venkateswararao, V. Joseph, S. Kumar, J.-H. Jou,Org. Electron., 2019, 64, 266-273. DOI:10.1016/j.orgel.2018.10.029
Effect of electron rich π-linkers on the functional properties of dyes featuring dithieno[3,2-b:2',3'-d]pyrrole donor, S. Kumar, K. R. J. Thomas, C.-T. Li, M.-S. Fan, K.-C. Ho, Dyes Pigm., 2019, 160, 614-623. DOI:10.1016/j.dyepig.2018.08.035

Design-to-Device Approach Affords Panchromatic Co-Sensitized Solar Cells, C. B. Cooper, E. J. Beard, Alvaro Vazquez-Mayagoitia, L. Stan, G. B. G. Stenning, D. W. Nye, J. A. Vigil, T. Tomar, J. Jia, G. B. Bodedla, S. Chen, L. Gallego, S. Franco, A. Carella, K. R. J. Thomas, S.Xue, X. Zhu, J. M. Cole, Adv. Energy Mater., 2018, 42, 1802820. DOI: 10.1002/aenm.201802820
Enabling a 6.5% external quantum efficiency deep-blue organic light-emitting diode with a solution-processable carbazole based emitter, J.-H. Jou, J.-L. Li, S. Sahoo, D. K. Dubey, R. A. K. Yadav, V. Joseph, K. R. J. Thomas, C.-W. Wang, J. Jayakumar, C.-H. Cheng, J. Phys. Chem. C, 2018, 42, 24295-24303. DOI: 10.1021/acs.jpcc.8b07641
Vinyl-Linked Cyanocarbazole-Based Emitters: Effect of Conjugation and Terminal Chromophores on the Photophysical and Electroluminescent Properties, V. Joseph, K. R. J. Thomas, J.-L. Li, S. Sahoo, M. Singh, J.-H. Jou, ACS Omega, 2018, 3, 16477-16488. DOI: 10.1021/acsomega.8b02198
Synthesis and characterization of naphthalimide-based dyes for dye sensitized solar cells, A. Saini, K. R. J. Thomas, Y.-J. Huang, K.-C. Ho, J. Mater. Chem. Mater. Electron., 2018, 10, 24013-24027. DOI: 10.1007/s10854-018-9750-4
Wide Color Gamut Deep-Blue OLED Architecture for Display Application, D. K. Dubey, R. K. Konidena, S. Sahoo, R. A. K. Yadav, S. S. Swayamprabha, K. R. J. Thomas and J.-H. Jou, ECS Trans., 2018, 85, 33-39. DOI: 10.1149/08507.0033ecst
Tuning the Photophysical and Electroluminescence Properties in Asymmetrically Tetrasubstituted Bipolar Carbazoles by Functional Group Disposition, R. K. Konidena, K. R. J. Thomas, A. Pathak, D. K. Dubey, S. Sahoo, and J.-H. Jou, ACS Appl. Mater. Interfaces, 2018, 10, 24013-24027. DOI: 10.1021/acsami.8b04566
Cyano-functionalized carbazole substituted pyrene derivatives for promising organic light-emitting diodes, V. Joseph, K. R. J. Thomas, S. Sahoo, M. Singh, and J.-H. Jou, Dyes Pigm., 2018, 158, 295-305. DOI: 10.1016/j.dyepig.2018.05.038
Tetra-substituted dipolar carbazoles: Tuning optical and electroluminescence properties by linkage variation, V. Joseph, K. R. J. Thomas, W. Y. Yang, R. A. K. Yadav, D. K. Dubey, and J.-H. Jou, Asian J. Org. Chem., 2018, 7, 1654-1666. DOI: 10.1002/ajoc.201800248
Highly efficient deep-blue organic light emitting diode with a carbazole based fluorescent emitter, S. Sahoo, D. K. Dubey, M. Singh, V. Joseph, K. R. J. Thomas and J.-H. Jou, Jpn. J. Appl. Phys., 2018, 57, 04FL08. DOI: 10.7567/JJAP.57.04FL08
Simple carbazole based deep-blue emitters: The effect of spacer, linkage and end-capping cyano group on the photophysical and electroluminescent properties, V. Joseph, K. R. J. Thomas, S. Sahoo, M. Singh, J.-H. Jou, Dyes Pigm., 2018, 151, 310-320. DOI: 10.1016/j.dyepig.2017.12.061
T-Shaped Benzimidazole Derivatives as Blue-Emitting Materials: Role of C2 Substituents on Photophysical Properties, K. R. J. Thomas and G. B. Bodedla, Asian J. Org. Chem., 2018, 7, 729-738. DOI: 10.1002/ajoc.201800003
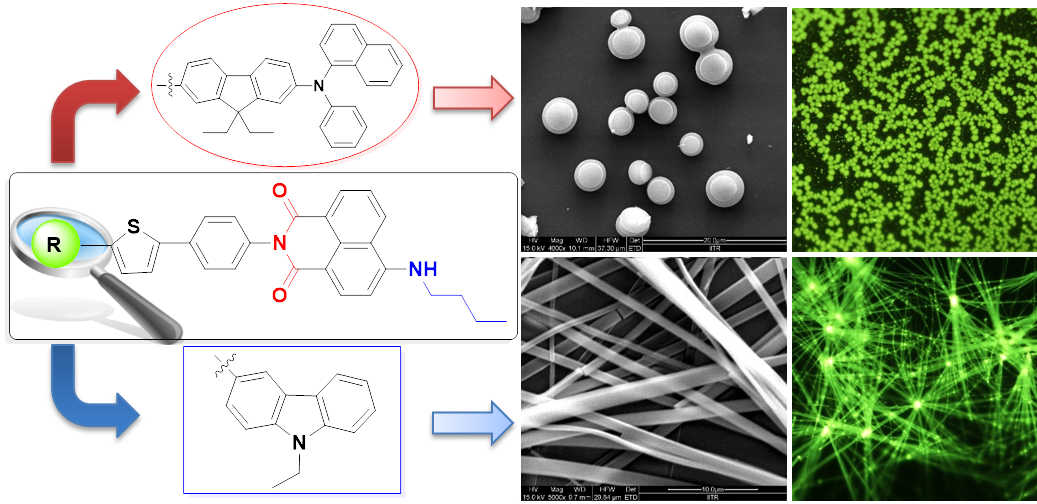
Manipulation of donor-acceptor interactions in carbazole-based emitters by chromophore choice to achieve near-UV emission, V. Joseph, K. R. J. Thomas, M. Singh, S. Sahoo, J.-H. Jou, Eur. J. Org. Chem., 2017, 6660-6670. DOI: 10.1002/ejoc.201701285
Fine-Tuning of Photophysical and Electroluminescence Properties of Benzothiadiazole-Based Emitters by Methyl Substitution, A. Pathak, K. R. J. Thomas, M. Singh, J.-H. Jou, J. Org. Chem., 2017, 82, 11512-11523. DOI: 10.1021/acs.joc.7b02127
New Molecular Design Based on Hybridized Local and Charge Transfer Fluorescence for Highly Efficient (> 6%) Deep-Blue Organic Light Emitting Diodes, R. K. Konidena, K. R. J. Thomas, D. K. Dubey, S. Sahoo, J.-H. Jou, Chem. Commun., 2017, 53, 11802-11805. DOI: 10.1039/C7CC07139F
Star-Shaped Asymmetrically Substituted Blue Emitting Carbazoles: Synthesis, Photophyscial, Electrochemical and Theoretical Investigations, R. K. Konidena, K. R. J. Thomas, ChemistrySelect, 2017, 2, 7514-7524. DOI: 10.1002/slct.201701336
Effect of electron-deficient linkers on the physical and photovoltaic properties of dithienopyrrole-based organic dyes, S. Kumar, K. R. J. Thomas, C.-T. Li and K.-C. Ho, J. Mater. Sci. Mater. Electron., 2017, 28 18404-18417. DOI: 10.1007/s10854-017-7787-4
Photophysics, electrochemistry, morphology and bioimaging applications of new 1,8-naphthalimide derivatives containing different chromophores, A. Saini, K. R. J. Thomas, A. Sachdev and P. Gopinath, Chem. Asian J., 2017, 12, 2612-2622. DOI: 10.1002/asia.201700968
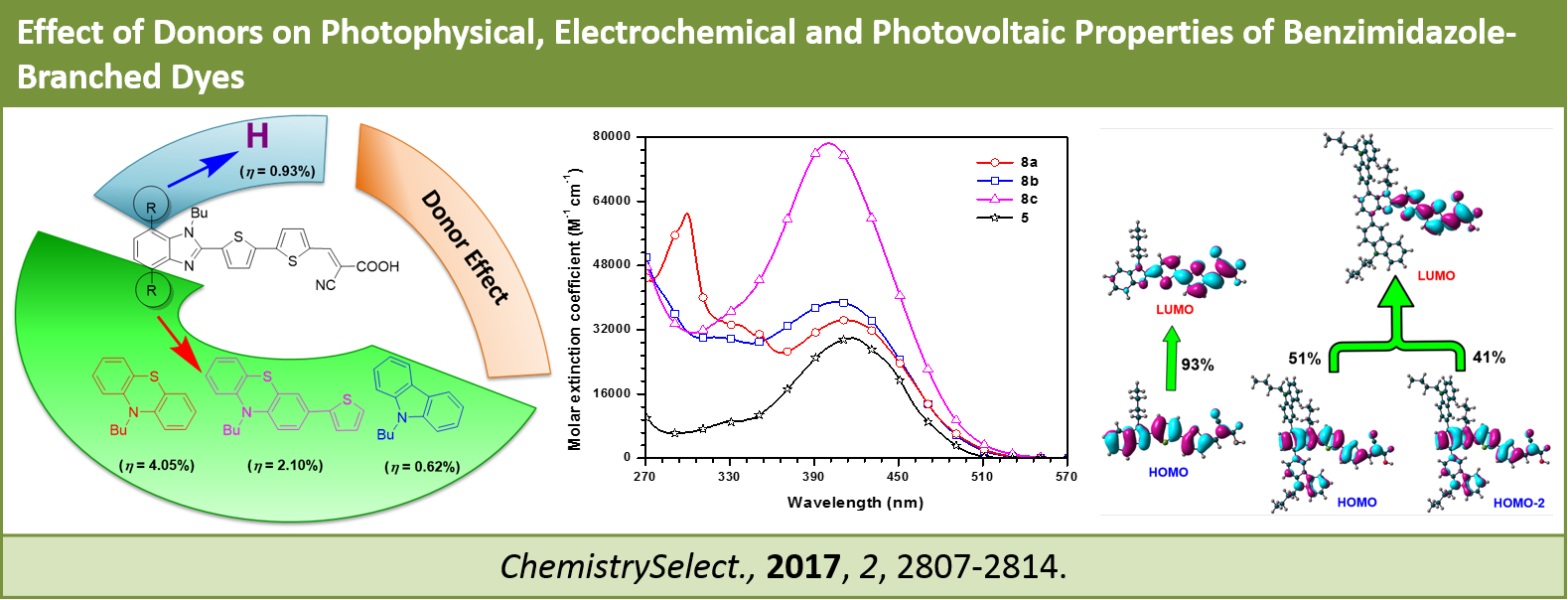
Effect of Donors on Photophysical, Electrochemical and Photovoltaic Properties of Benzimidazole-Branched Dyes, G. B. Bodedla, K. R. J. Thomas, M.-S. Fan and K.-C. Ho, ChemistrySelect, 2017, 2, 2807-2814. DOI: 10.1002/slct.201700014
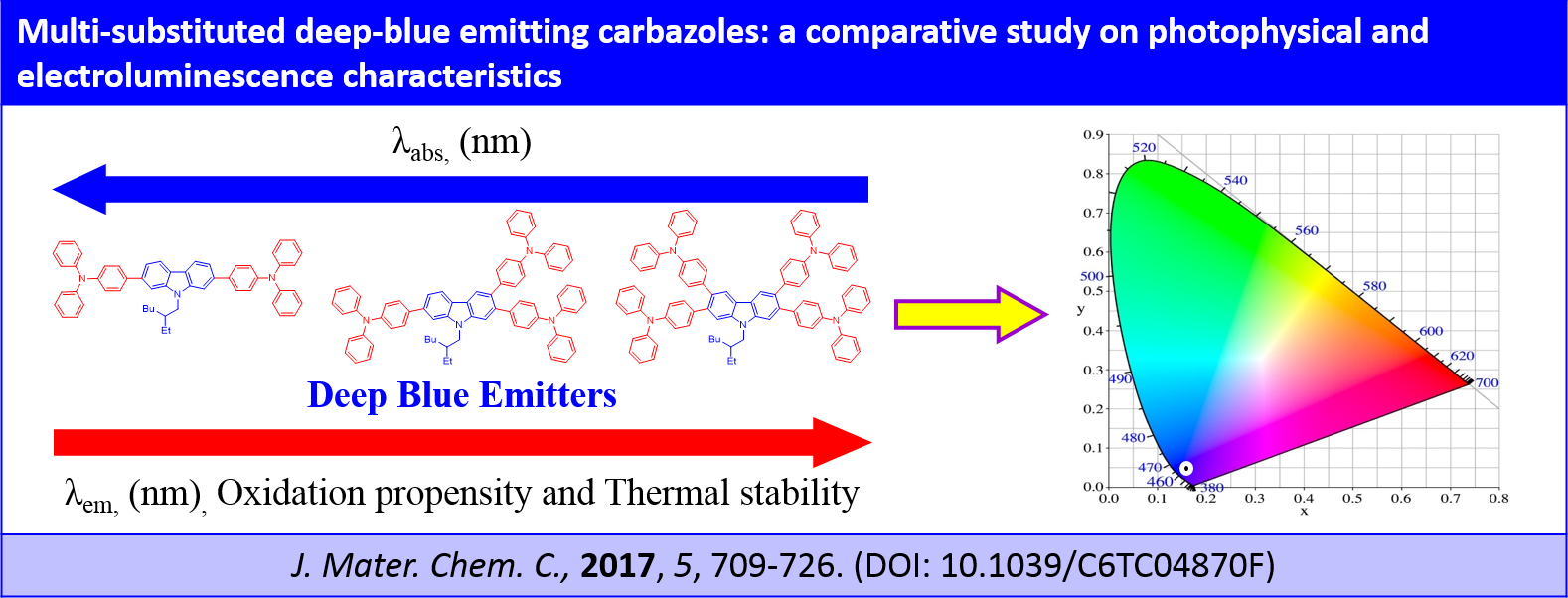
Multi-substituted deep-blue emitting carbazoles: a comparative study on photophysical and electroluminescence characteristics, R. K. Konidena, K. R. J. Thomas, S. Sahoo, D. K. Dubey, J.-H. Jou, J. Mater. Chem. C, 2017, 5, 709-726. DOI: 10.1039/C6TC04870F
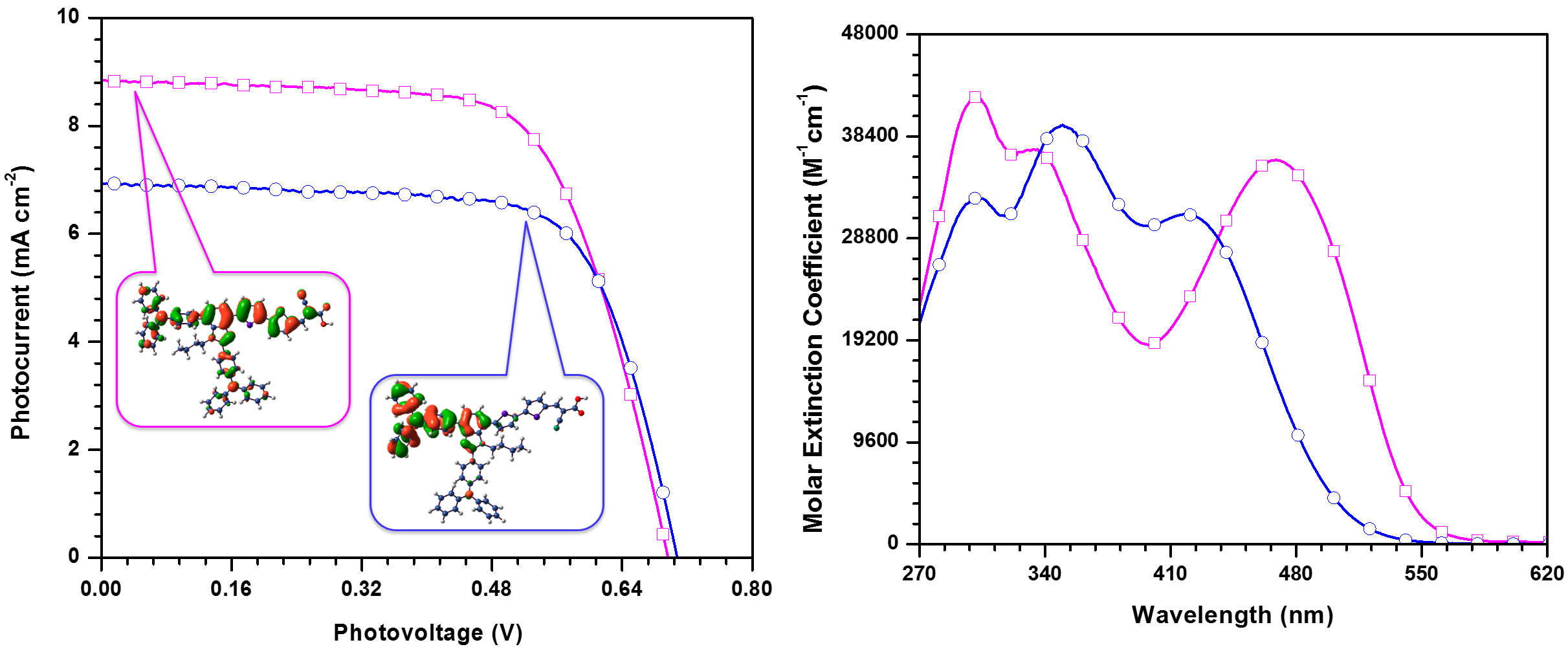
Organic dyes containing fluorenylidene functionalized phenothiazine donors as sensitizers for dye sensitized solar cells, A. Saini, K. R. J. Thomas, C.-T. Li and K.-C. Ho, J. Mater. Sci.: Mater. Electron., 2016, 27, 12392-12404.
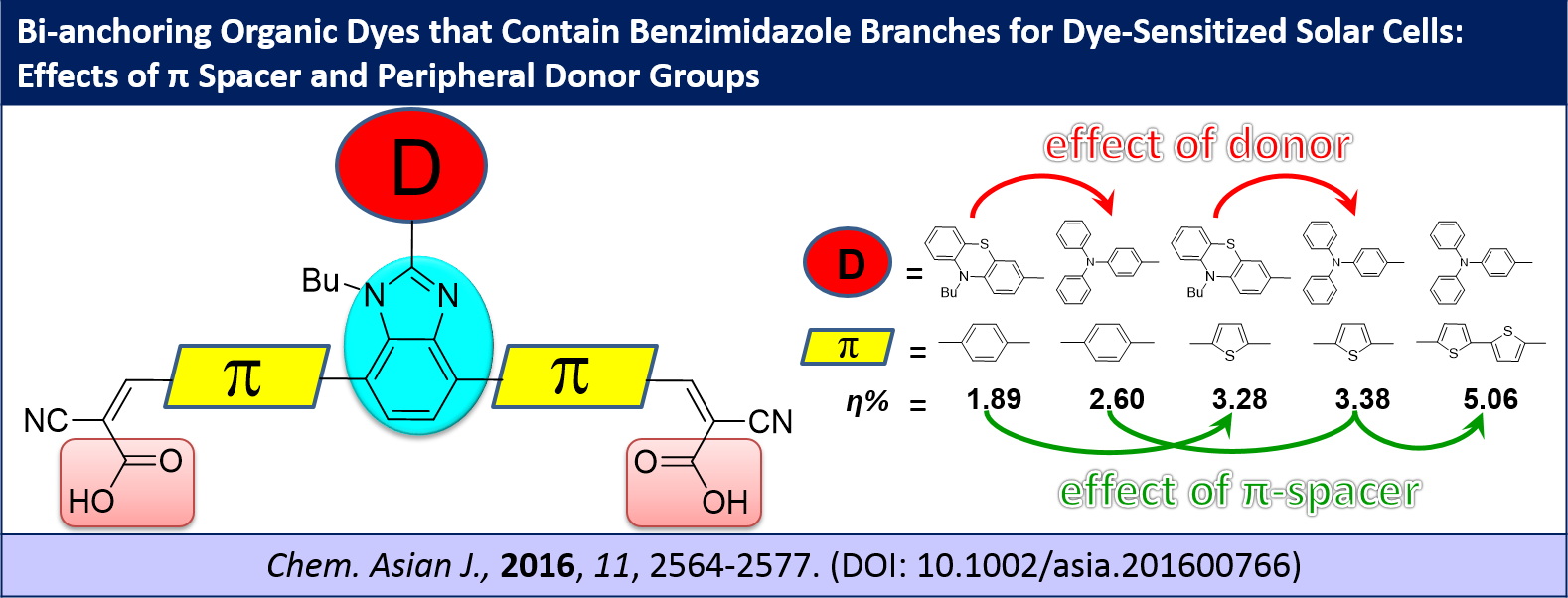
Bi-anchoring organic dyes that contain benzimidazole branches for dye-sensitized solar cells: effects of π spacer and peripheral donor groups, G. B. Bodedla, K. R. J. Thomas, M.-S. Fan and K.-C. Ho, Chem. Asian J., 2016, 11, 2564-2577.
9,9-Diethyl-7-ethynyl-N,N-diphenyl-9H-fluoren-2-amine, J. J. Novina, G. Vasuki, P. Singh and K. R. J. Thomas, IUCrData, 2016, 1, x162006.
Bis-naphthalimides bridged by electron acceptors: optical and self-assembly characteristics, A. Saini and K. R. J. Thomas, RSC Adv., 2016, 6, 71638-71651.
Synthesis, characterization and electroluminescence of carbazole-benzimidazole hybrids with thiophene/phenyl linker, D. Karthik, K.R. J. Thomas, J.-H. Jou and Y.-L. Chen, Dyes Pigm., 2016, 133, 132-142.
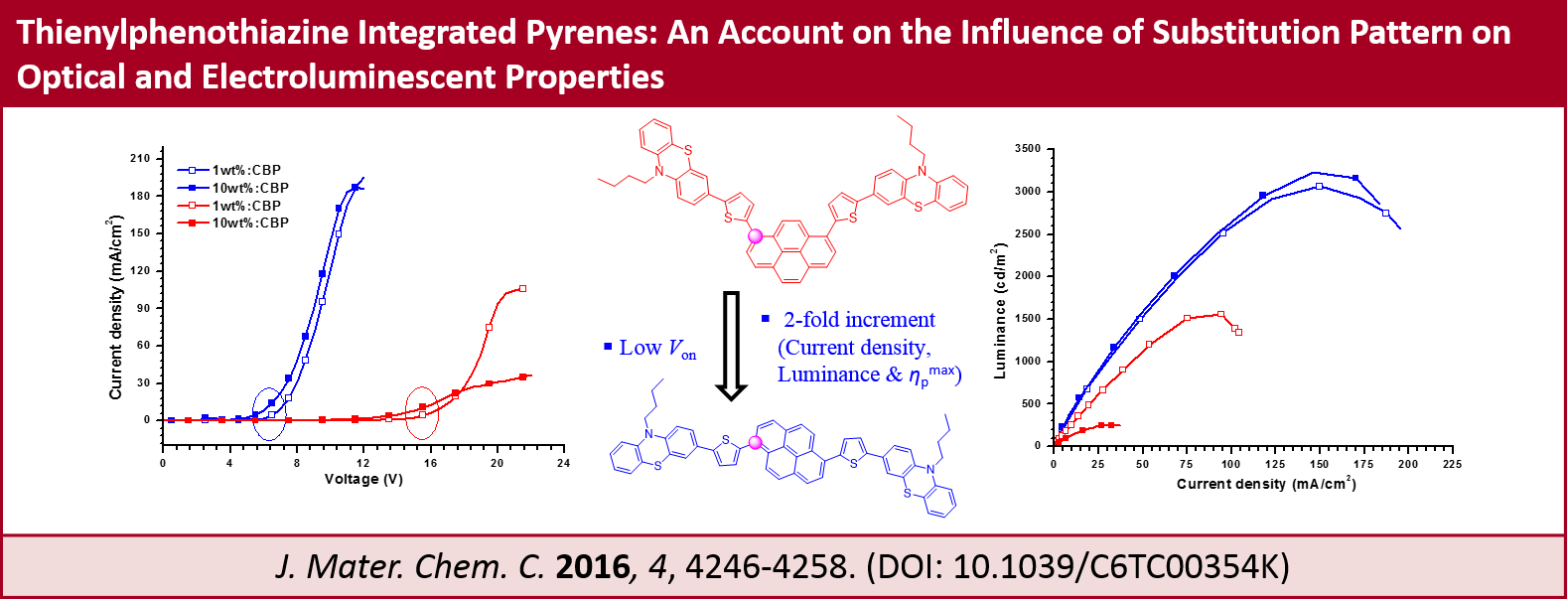
Thienylphenothiazine Integrated Pyrenes: An Account on the Influence of Substitution Pattern on Optical and Electroluminescent Properties, R. K. Konidena, K. R. J. Thomas, M. Singh and J.-H. Jou, J. Mater. Chem. C, 2016, 4, 4246-4258.
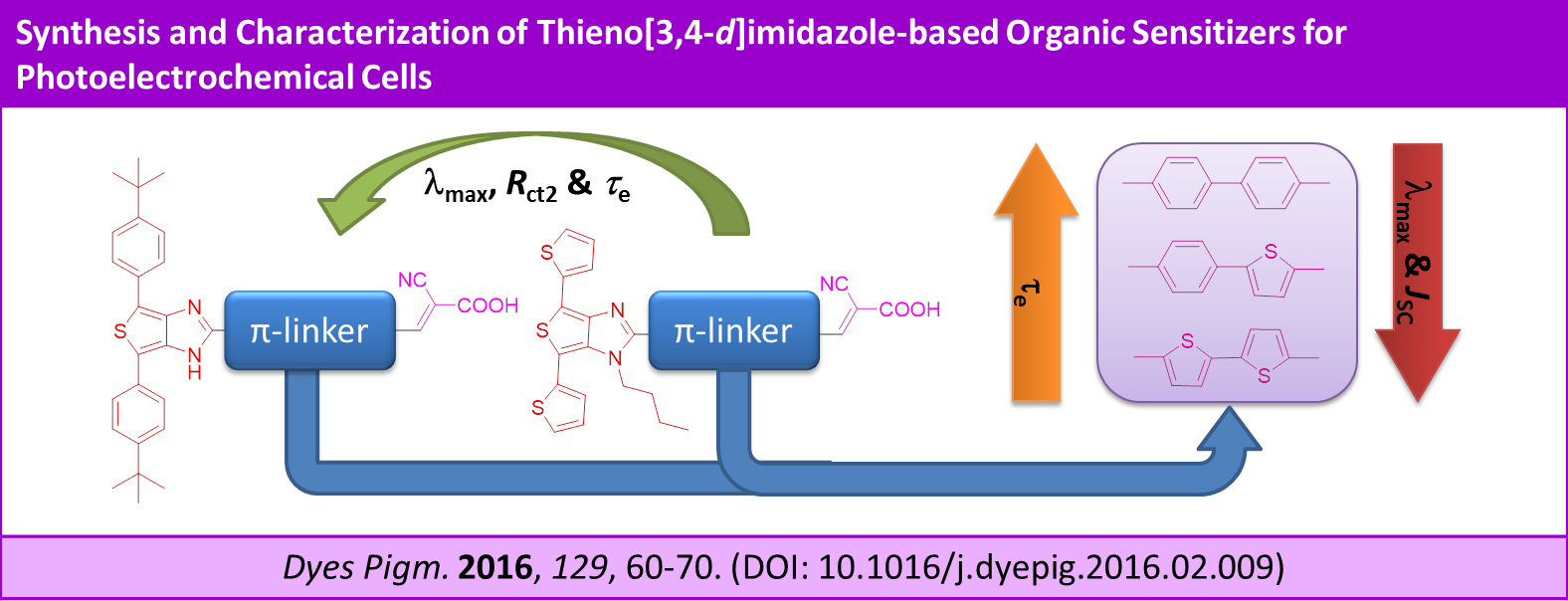
Synthesis and characterization of thieno[3,4-d]imidazole-based organic sensitizers for photoelectrochemical cells, D. Karthik, V. Kumar, K. R. J. Thomas, C.-T. Li and K.-C. Ho, Dyes Pigm., 2016, 129, 60-70.
Benzimidazole-Branched Isomeric Dyes: Effect of Molecular Constitution on Photophysical, Electrochemical and Photovoltaic Properties, G. B. Bodedla, K. R. J. Thomas, M.-S. Fan and K.-C. Ho, J. Org. Chem., 2016, 81, 640-653.
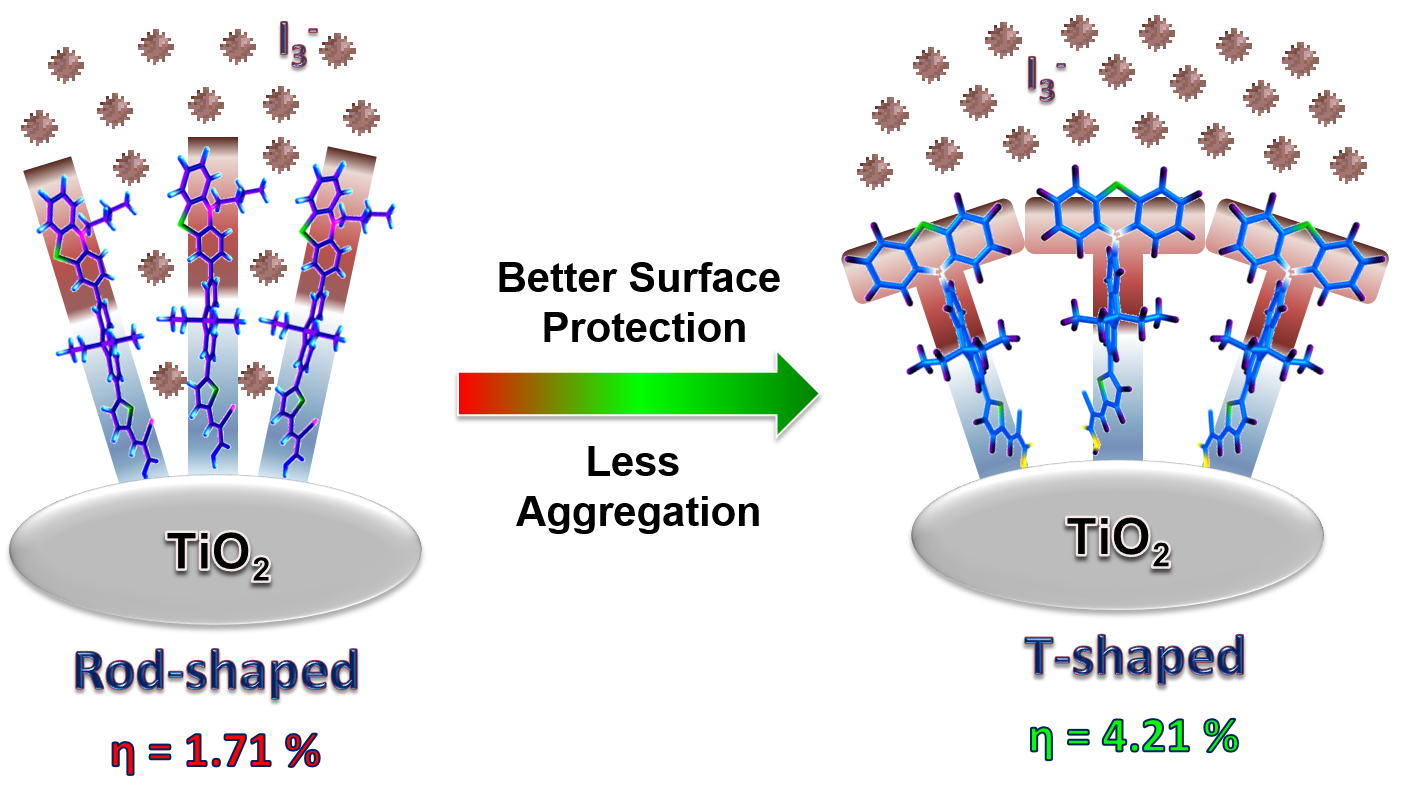
Phenothiazine-based bipolar green-emitters containing benzimidazole units: synthesis, photophysical and electroluminescence properties, G. B. Bodedla, K. R. J. Thomas, S. Kumar, J.-H. Jou and C.-J. Li, RSC Adv., 2015, 5, 87416-87428.
Organic dyes containing fluoreneamine donor and carbazole π-linker for dye-sensitized solar cells, K. R. J. Thomas, A. Venkateswararao, C.-P.Lee and K.-C. Ho, Dyes Pigm., 2015, 126, 154-165.
Synthesis and photovoltaic properties of organic dyes containing N-fluoren-2-yl dithieno[3,2-b:2′,3′-d]pyrrole and different donors, S. Kumar, K. R. J. Thomas, C.-T. Li, K.-C. Ho, Org. Electron., 2015, 26, 109-116.
Plant growth absorption spectrum mimicking light sources, J.-H. Jou, C.-C. Lin, T.-H. Li, C.-J. Li, S.-H. Peng, F.-C. Yang, K. R. J. Thomas, D. Kumar, Y. Chi and B.-D. Hsu, Materials, 2015, 8, 5265-5275.
Benzothiadiazole-based organic dyes with pyridine anchors for dye-sensitized solar cells: effect of donor on optical properties, M. N. K. P. Bolisetty, C.-T. Li, K. R. J. Thomas, G. B. Bodedla and K.-C. Ho, Tetrahedron, 2015, 71, 4203-4212.
Phenothiazine Decorated Carbazoles: Effect of Substitution Pattern on the Optical and Electroluminescent Characteristics, R. K. Konidena, K. R. J. Thomas, S. Kumar, Y.-C. Wang, C.-J. Li and J.-H. Jou, J. Org. Chem., 2015, 80, 5812-5823.
Highly efficient ultra-deep blue organic light-emitting diodes with a wet- and dry-process feasible cyanofluorene acetylene based emitter, J.-H. Jou, S. Kumar, P.-H. Fang, A. Venkateswararao, K. R. J. Thomas, J.-J. Shyue, Y.-C. Wang, T.-H. Li and H.-H. Yu, J. Mater. Chem. C, 2015, 3, 2182-2194.
Fluorene-Based Sensitizers with a Phenothiazine Donor: Effect of Mode of Donor Tethering on the Performance of Dye-Sensitized Solar Cells, A. Baheti, K. R. J. Thomas, C.-T. Li, C.-P. Lee and K.-C. Ho, ACS Appl. Mater. Interfaces, 2015, 7, 2249-2262.
Triarylamine-Free Pyrenoimidazole-Containing Organic Dyes with Different π-Linkers for Dye-Sensitized Solar Cells, D. Kumar, K. R. J. Thomas, C.-P. Lee and K.-C. Ho, Asian J. Org. Chem., 2015, 4, 164-172.
Synthesis, optical, electrochemical and photovoltaic properties of organic dyes containing trifluorenylamine donors, A. Baheti, S. R. Gajjela, P. Balaya and K. R. J. Thomas, Dyes Pigm., 2015, 113, 78-86.
Functional tuning of organic dyes containing 2,7-carbazole and other electron-rich segments in the conjugation pathway, A. Venkateswararao, K. R. J. Thomas, C.-T. Li and K.-C. Ho, RSC Adv., 2015, 5, 17953-17966.
Deep-blue emitting pyrene-benzimidazole conjugates for solution processed organic light-emitting diodes, D. Karthik, K. R. J. Thomas, J.-H. Jou, S. Kumar, Y.-L. Chen and Y.-C. Jou, RSC Adv., 2015, 5, 8727-8738.
Effect of Auxiliary Chromophores on the Optical, Electrochemical, and Photovoltaic Properties of Carbazole-Based Dyes, A. Venkateswararao, K. R. J. Thomas, C.-P. Lee and K.-C. Ho, Asian J. Org. Chem., 2015, 4, 69-80.
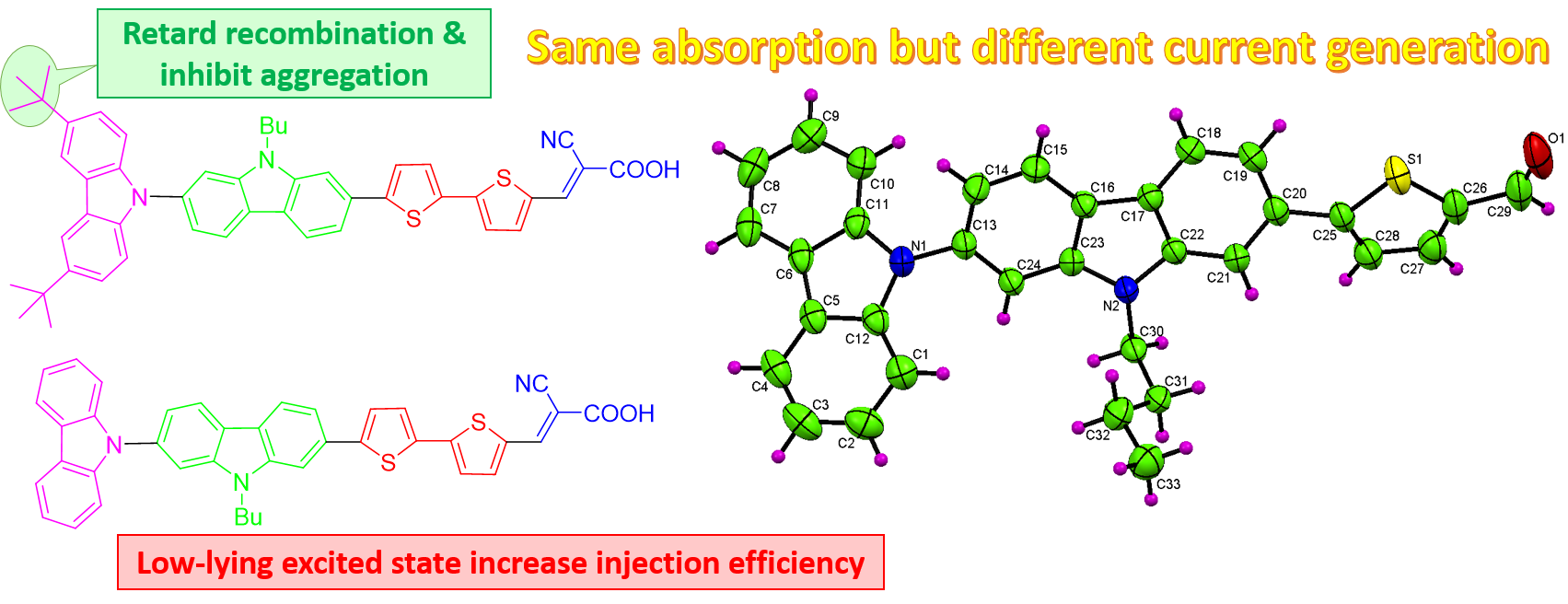
Organic dyes containing indolo[2,3-b]quinoxaline as a donor: synthesis, optical and photovoltaic properties, A. Venkateswararao, P. Tyagi, K. R. J. Thomas, P.-W. Chen and K.-C. Ho, Tetrahedron, 2014, 70, 6318-6327.
Monoanchoring (D-D-π-A) and Dianchoring (D-D-(π-A)(2)) Organic Dyes Featuring Triarylamine Donors Composed of Fluorene and Carbazole, A. Baheti, K. R. J. Thomas, L.-C. Lin, K. M. Lee, Asian J. Org. Chem., 2014, 3, 886-898.
2-Hydroxyarylimidazole-based colorimetric and ratiometric fluoride ion sensors, D. Kumar and K. R. J. Thomas, RSC Adv., 2014, 4, 56466-56474.
Functional tuning of phenothiazine-based dyes by a benzimidazole auxiliary chromophore: an account of optical and photovoltaic studies, G. B. Bodedla, K. R. J. Thomas, C.-T. Li and K.-C. Ho, RSC Adv., 2014, 4, 53588-53601.
Materials, Designs, Fabrications, and Applications of Organic Electronic Devices (Editorial), J.-W. Jou, R. Lygaitis, K. R. J. Thomas and L.-S. Liao, Int. J. Photoenergy, 2014, 512717.
Organic dyes containing fluoren-9-ylidene chromophores for efficient dye-sensitized solar cells, A. Baheti, K. R. J. Thomas, C.-P.Lee, C.-T. Li, and K.-C. Ho, J. Mater. Chem. A, 2014, 2, 5766-5779.
Selective naked-eye cyanide detection in aqueous media using a carbazole-derived fluorescent dye, R. K. Konidena and K. R. J. Thomas, RSC Adv., 2014, 4, 22902-22910.
Organic Dyes Containing Fluorene Decorated with Imidazole Units for Dye-Sensitized Solar Cells, D. Kumar, K. R. J. Thomas, C.-P.Lee and K.-C. Ho, J. Org. Chem., 2014, 79, 3159–3172.
Organic Dyes Containing Carbazole as Donor and π-Linker: Optical, Electrochemical, and Photovoltaic Properties, A. Venkateswararao, K. R. J. Thomas, C.-P.Lee, C.-T. Li, and K.-C. Ho, ACS Appl. Mater. Interfaces, 2014, 6, 2528-2539.
Synthesis and characterization of polybrominated fluorenes and their conversion to polyphenylated fluorenes and cyclopenta[def]triphenylene, S. Kumar, D. Karthik, K.R.J. Thomas and M. S. Hundal, Tetrahedron Lett., 2014, 55, 1931-1935.
Insights into the Co-sensitizer Adsorption Kinetics for Complementary Organic Dye-sensitized Solar Cells, L.-Y. Lin, M.-H. Yeh, C.-P. Lee, J. Chang, A. Baheti, R. Vittal, K.R.J. Thomas, and K.-C. Ho, J. Power Sources, 2014, 247, 906-914.
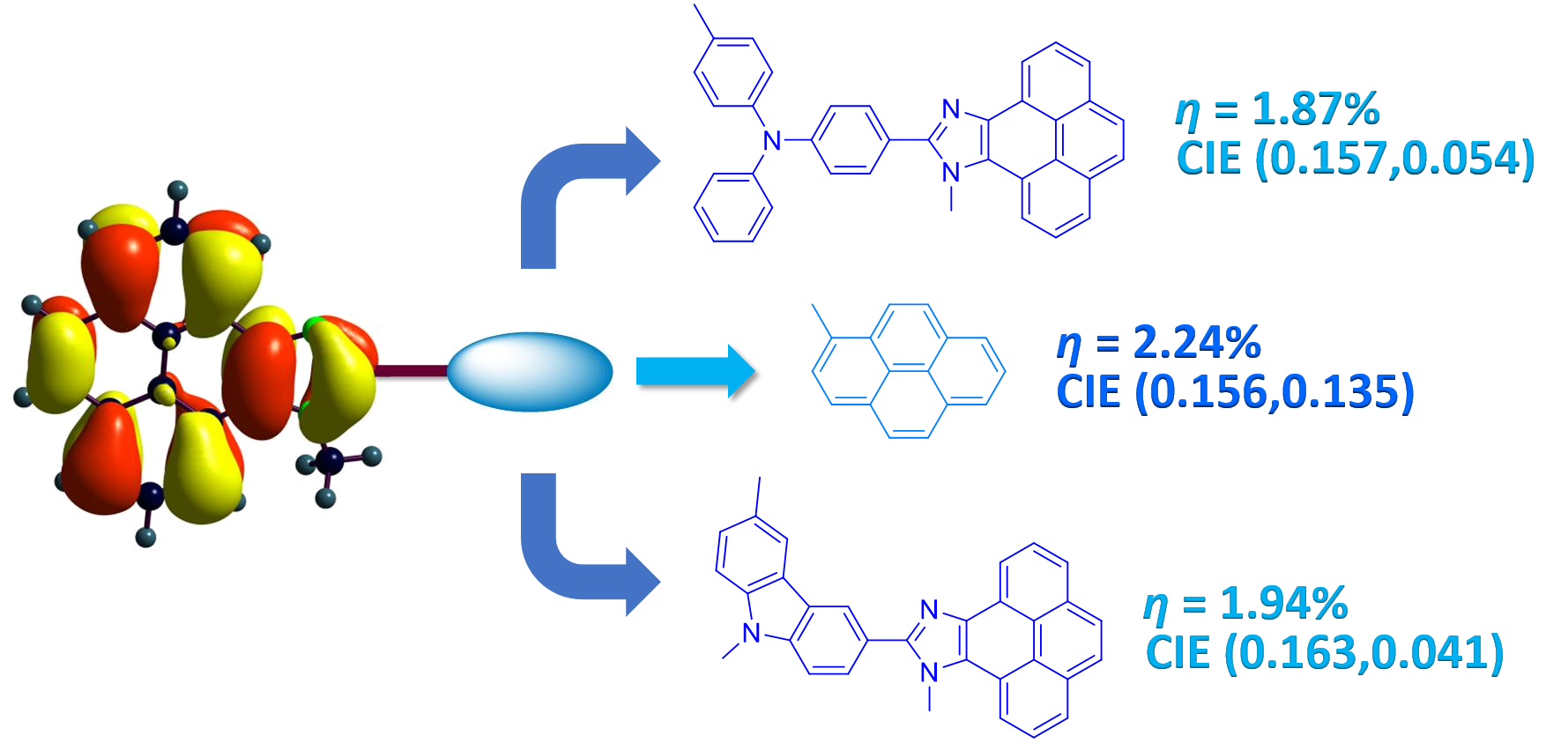
Synthesis and characterization of dianchoring organic dyes containing 2,7-diaminofluorene donors as efficient sensitizers for dye-sensitized solar cells, A. Baheti, K. R. J. Thomas, C.-P. Lee and K.-C. Ho, Org. Electron., 2013, 14, 3267-3276.
Pyrenoimidazole-based deep blue emitting materials: Optical, electrochemical and electroluminescent characteristics, D. Kumar, K. R. J. Thomas, Ching-Chiao Lin and Jwo-Huei Jou, Chem. Asian J., 2013, 8, 2111-2124.
Synthesis and characterization of organic dyes containing 2,7-disubstituted carbazole π-linker, A. Venkateswararo, K. R. J. Thomas C.-P. Lee and K.-C. Ho, Tetrahedron Lett., 2013, 54, 3985-3989.
Co-Sensitization Promoted Light Harvesting for Organic Dye-Sensitized Solar Cells Using Unsymmetrical Squaraine Dye and Novel Pyrenoimidazole-Based Dye, J. Chang, C.-P. Lee, D. Kumar, P.-W. Chen, L.-Y. Lin, K. R. J. Thomas and K.-C. Ho, J. Power Sources, 2013, 240, 779-785.
Electroanalytical performance of Cd(II) selective sensor based on PVC membranes of 5,5-(5,5-(benzo[c][1,2,5]thiadiazole-4,7-diyl)bis(thiophene-5,2-diyl))bis(N1,N1,N3,N3-tetraphenylbenzene-1,3-diamine), A. K. Singh, A. K. Jain, A. Uphadhyay, K. R. J. Thomas, P. Singh, Int. J. Environ. Anal. Chem., 2013, 93, 813-827.
Synthesis, Optical Properties and Blue Electroluminescence of Fluorene Derivatives Containing Multiple Imidazoles Bearing Polyaromatic Hydrocarbons, D. Kumar, K. R. J. Thomas, Y.-L. Chen, Y.-C. Jou and J.-H. Jou, Tetrahedron, 2013, 69, 2594-2602.
A new porphyrin bearing a pyridinylethynyl group as sensitizer for dye sensitized solar cells, D. Daphnomili, G.D. Sharma, S. Biswas, K.R. J. Thomas and A.G. Coutsolelos, J. Photochem. Photobiol., A Chem., 2013, 253, 88-96.
N-(4,4'-Dibromo-[1,1'-biphenyl]-2-yl)benzamide, J. J. Novina, G. Vasuki, A. Baheti and K. R. J. Thomas, Acta Cryst., 2013, E69, o222.
Fluorene based organic dyes for dye sensitized solar cells: structure-property relationships, K. R. J. Thomas and A. Baheti, Mater. Technol., 2013, 28, 71-87.
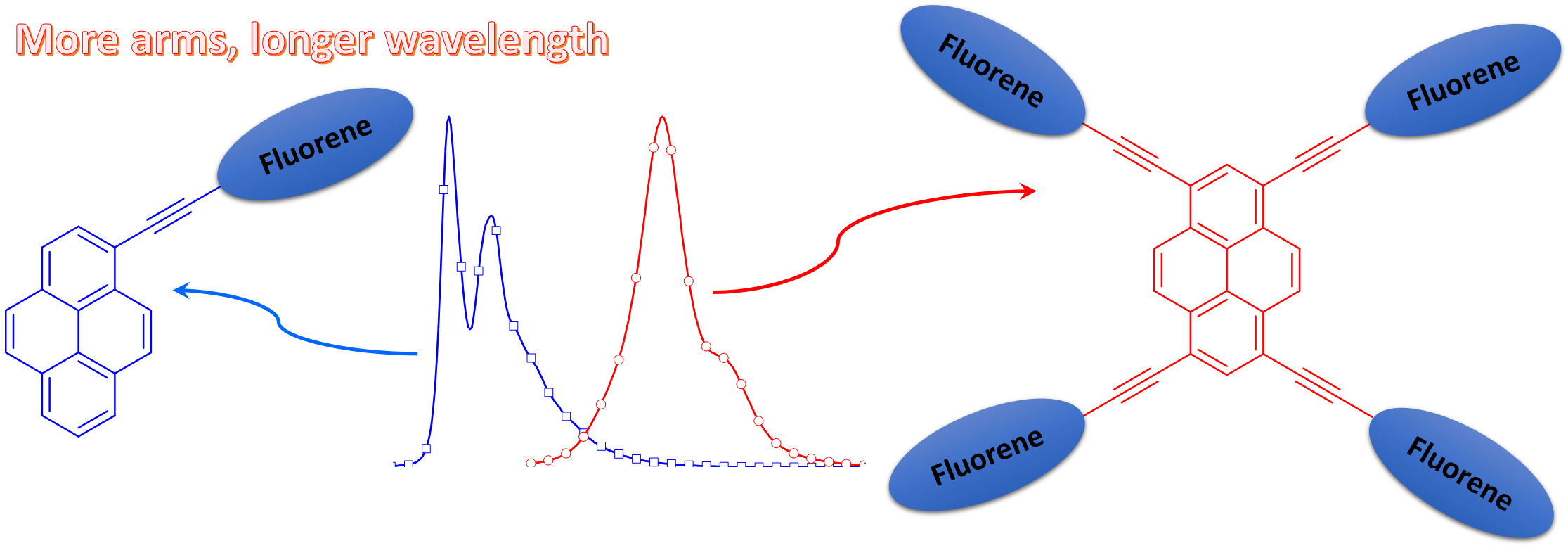
Fine Tuning of DSSC Performance by Variation of π-Spacers in Organic Dyes Containing 2,7-Diaminofluorene Donor, A. Baheti, K. R. J. Thomas, C.-P. Lee and K.-C. Ho, Chem. Asian. J., 2012, 7, 2942-2954.
Fluorene-based organic dyes containing acetylene linkage for dye-sensitized solar cells, P. Singh, A. Baheti, K. R. J. Thomas, C.-P. Lee and K.-C. Ho, Dyes Pigm., 2012, 95, 523-533.
The use of a polarity matching and high energy exciton generating host in fabricating efficient purplish-blue OLEDs from a sky-blue emitter, J.-H. Jou, Y.-L. Chen, J.-R. Tseng, R.-Z. Wu, J.-J. Shyue, K. R. J. Thomas, N. Kapoor, C.-T. Chen, Y.-P. Lin, P.-H. Wang, H.-W. Hung and S.-P. Chen, J. Mater. Chem., 2012, 22, 15500-15506.
Organic dyes containing pyrenylamine-based cascade donor systems with different aromatic π-linkers for dye-sensitized solar cells: optical, electrochemical, and device characteristics, K. R. J. Thomas, N. Kapoor, C.-P. Lee and K.-C. Ho, Chem. Asian J., 2012, 7, 738-750.
Pyrene-fluorene hybrids containing acetylene linkage as color-tunable emitting materials for organic light-emitting diodes, K. R. J. Thomas, N. Kapoor, M. N. K. P. Bolisetty, J.-H. Jou, Y.-L. Chen and Y.-C. Jou, J. Org. Chem., 2012, 77, 3921-3932.
A novel 2,7-diaminofluorene-based organic dye for a dye-sensitized solar cell, L.-Y. Lin, C.-P. Lee, M.-H. Yeh, A. Baheti, R. Vittal, K. R. J. Thomas and K.-C. Ho, J. Power. Sources, 2012, 215, 122-129.
Synthesis and characterization of a new perylenebisimide (PBI) derivative and its application as electron acceptor for bulk heterojunction polymer solar cells, G.D. Sharma, M.S. Roy, J.A. Mikroyannidis, K.R. J. Thomas, Org. Electron., 2012, 13, 3118-3129.
Dithienylthienothiadiazole-based organic dye containing two cyanoacrylic acid anchoring units for dye-sensitized solar cells, G. D. Sharma, J. A. Mikroyannidis, M. S. Roy, K. R. J. Thomas, R. J. Ball, and R. Kurchania, RSC Adv., 2012, 2, 11457-11464.
Organic bulk heterojunction solar cells based on solution processable small molecules (A-π-A) featuring 2-(4-nitrophenyl) acrylonitrile acceptors and phthalimide-based π-linkers, G. D. Sharma, J. A. Mikroyannidis, R. Kurchania and K. R. J. Thomas, J. Mater. Chem., 2012, 22, 13986-13995.
A new family of A2B2 type porphyrin derivatives: Synthesis, Physicochemical Characterization and their Application in Dye-Sensitized Solar Cell, M. Panda, G. D. Sharma, K. R. J. Thomas and A. G. Coutsolelos, J. Mater. Chem., 2012, 22, 8092-8102.
New Triphenylamine (TPA) Based Organic Dyes with Different Number of Anchoring Groups for Dye-Sensitized Solar Cells, S. P. Singh, M. S. Roy, K. R. J. Thomas, S. Balaiah, K. Bhanuprakash and G. D.Sharma, J. Phys. Chem. C, 2012, 116, 5941-5950.
Efficient bulk heterojunction photovoltaic devices based on diketopyrrolopyrrole containing small molecule as donor and modified PCBM derivatives as electron acceptors, G.D. Sharma, J.A. Mikroyannidis, S.S. Sharma, M.S. Roy and K.R. J. Thomas, Org. Electron., 2012, 13, 652-666.
Bulk heterojunction organic photovoltaic devices based on small molecules featuring pyrrole and carbazole and 2-(4-nitrophenyl)acrylonitrile acceptor segments as donor and fullerene derivatives as acceptor, G. D. Sharma, J. A. Mikroyannidis, S. S. Sharma and K. R. J. Thomas, Dyes Pigm., 2012, 94, 320-329.
2,2'-Bithiophene-3,3'-dicarbonitrile, J. J. Novina, G. Vasuki, D. Karthik and K. R. J. Thomas, Acta Cryst., 2012, E68, o2542.
N,N-Dimethyl-4-[(E)-2-(3,6,7-tribromo-9-butyl-9H-carbazol-2-yl)ethenyl]aniline, S. Kumar, K. R. J. Thomas, S. W. Ng and E. R. T. Tiekink, Acta Cryst., 2012, E68, o1121.
N-(9,9-Dipropyl-9H-fluoren-2-yl)-7-(piperidin-1-yl)-2,1,3-benzothiadiazol-4-amine, M. N. K. P. Bolisetty, S. Kumar, K. R. J. Thomas, S. W. Ng and E. R. T. Tiekink, Acta Cryst., 2012, E68, o911-o912.
N2-(7-Bromo-9-butyl-9H-carbazol-2-yl)-9,9-diethyl-N2,N7,N7-triphenyl-9H-fluorene-2,7-diamine, A. Baheti, K. R. J. Thomas, S. W. Ng and E. R. T. Tiekink, Acta Cryst., 2012, E68, o860-o861.
4,4'-Dibromo-2-nitrobiphenyl, J. Novina, G. Vasuki, S. Kumar and K. R. J. Thomas, Acta Cryst., 2012, E68, o319.